Latanoprost Drug: Indication, Dosage, Precaution, Side Effect , Storage, Category Type and corresponding Brands - www.genericdrugscan.com
Latanoprost
Drug Status in USA : ApprovedDrug Status in Canada : Approved
Indication : Why is this medication prescribed?
Reduction of elevated intra-ocular pressure in patients with primary open glaucoma and ocular hypertension.Contraindication: What are the special precautions to be followed?
Under following conditions, use of this medication is inadvisable. Its use should be withhold as it would cause harm to the patientHypersensitivity.
Other Precaution to be observed while taking this medication
Do not use with 5 minutes of thiomersal-containing preparations. Aphakia or pseudophakia with tom posterior lens cap or anterior chamber lenses, risk factors for cystoid macular oedema, brittle or severe asthma, history of intraocular inflammation, inflammatory, neovascular, angle-closure or congenital glaucoma, pregnancy, lactation. Remove contact lenses during use.What are possible side effects of this medication ?
Brown pigmentation particularly in those with mixed colour irides, blepharitis, ocular irritation and pain, darkening, thickening and lengthening of eyelashes, localised oedema, conjunctival hyperaemia, transient punctate epithelial erosions, dyspnoea, exacerbation of asthma, local skin reactions, iritis, uveitis, darkening of palpebral skin.Drug Category/Class
- Prostaglandin Analogues
- Antiglaucoma Preparations and Miotics
- Ophthalmologicals
- Sensory Organs
- Prostaglandin analogues
| Prescribed | For the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. |
| Weight : | 432.5928 |
| Structure | Latanoprost |
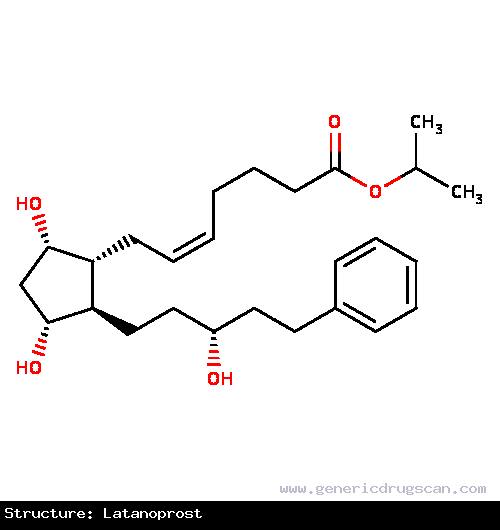 | |
| Formula | C26H40O5 |
Latanoprost has 9 Brands listed
| 9 PM (Eye) (2.5 ml) | Ioprost (Eye) (2.5 ml) |
| Lacoma (Eye) (2.5 ml) | Laprost (Eye) (3 ml) |
| Last Time (Eye) (2.5 ml) | Latochek (Eye) (2.5 ml) |
| Latodrops (Eye) (3 ml) | Latoprost (Eye) (2.5 ml) |
| Xalatan (Eye) (2.5 ml) |
Search Generic Drugs alphabetically
